Plasmodium falciparum is a highly lethal malaria parasite of humans. A major portion of its life cycle is dedicated to invading and multiplying inside erythrocytes. The molecular mechanisms of erythrocyte invasion are incompletely understood. P. falciparum depends heavily on sialic acid present on glycophorins to invade erythrocytes. However, a significant proportion of laboratory and field isolates are also able to invade erythrocytes in a sialic acid-independent manner. The identity of the erythrocyte sialic acid-independent receptor had been a mystery for decades. Our results showed that both sialic acid-dependent and independent strains interacted with CR1 in the normal red cell during the invasion process. However, only sialic acid-independent strains can do so without the presence of glycophorin sialic acid. Our results closed a longstanding and important gap in the understanding of the mechanism of erythrocyte invasion by P. falciparum that will eventually make possible the development of an effective blood stage vaccine.
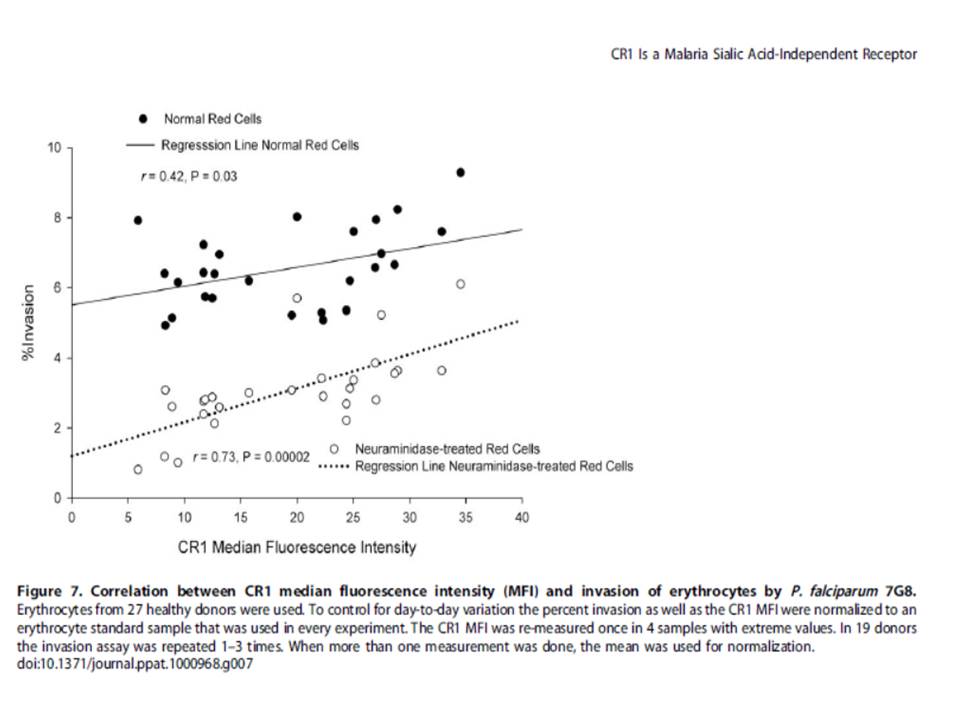
The sialic acid molecules in membrane-bound glycophorins are the preferred way used for invasion of erythrocytes by P. falciparum. Treatment with neuraminidase removes sialic acid. When testing human erythrocytes expressing different levels of CR1, the invasion of neuraminidase-treated erythrocytes correlates with the level of CR1 expression. Finally, both sialic acid-independent and dependent strains invade CR1 transgenic mouse erythrocytes preferentially over wild-type erythrocytes but invasion by the latter is more sensitive to neuraminidase.
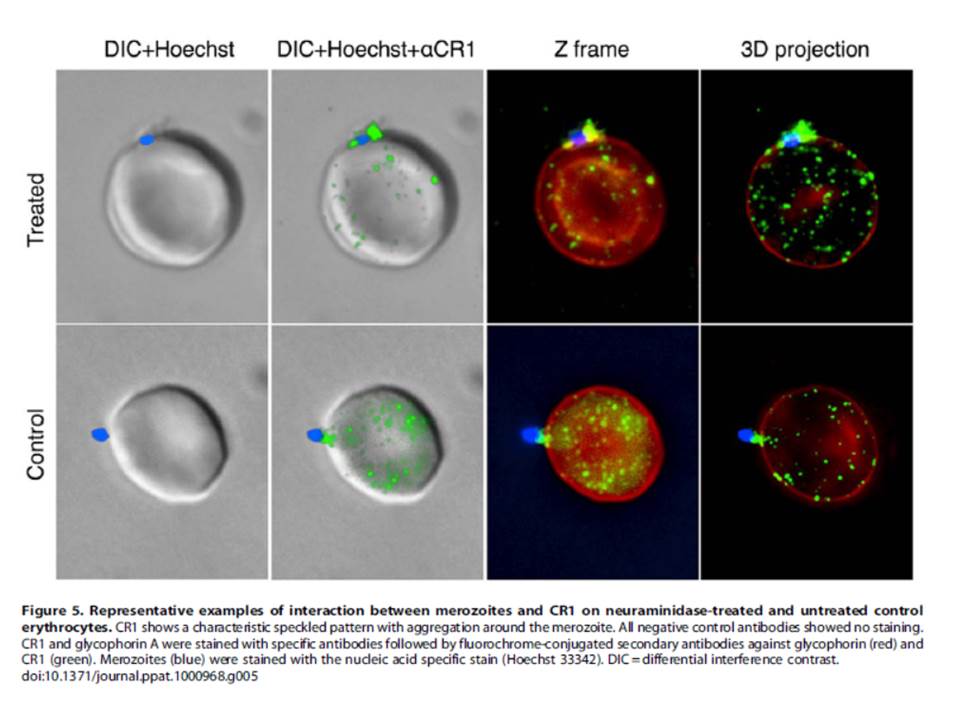
We showed that soluble CR1 (sCR1) as well as polyclonal and monoclonal antibodies against CR1 inhibit sialic acid-independent invasion in a variety of laboratory strains and wild isolates, and that merozoites interact directly with CR1 on the erythrocyte surface and with sCR1-coated microspheres.
Publications
Extracellular vesicles could carry an evolutionary footprint in interkingdom communication. Ricardo Correa, Zuleima Caballero, Luis Fernando De León and Carmenza Spadafora. Front Cell Infect Microbiol. 2020 Mar 3;10:76. doi: 10.3389/fcimb.2020.00076. eCollection 2020
